Relative strength of an acid ↑ (increases), its Ka ↑ (increases) and its pKa ↓(decreases).
(The Ka and pKa of an acid depend on the strength of an acid, but not its concentration.)
Relative strength of a base ↑ (increases), its Kb ↑ (increases) and its pKb ↓ (decreases).
(The Kb and pKb of an acid depend on the strength of an acid, but not its concentration.)
The stronger the acid, the more electrolytic it is, because it conducts electricity better due to the greater number of ions in solution.
The stronger the base, the more readily it undergoes hydrolysis when mixed with waterAcid Name pKa Cl3CCOOH Trichloroacetic acid 0.64 Cl2HCCOOH Dichloroacetic acid 1.27 H2SO3 Sulfurous acid 1.82 HClO2 Chloroacetic acid 1.90 ClH2CCOOH Chloroacetic acid 2.82 HF Hydrofluoric acid 3.15 HNO2 Nitrous acid 3.41 HCOOH Formic acid 3.74 H3CCOOH Acetic acid 4.74 2,4-(H3C)2C6H3NH3+ 2,4-dimethylanilinium 5.08 4-H2NC6H4NH3+ 4-aminoanilinium 6.18 H3CO3 Carbonic acid 6.36 4-O2NC6H4OH 4-nitrophenol 7.15 HClO Hypochlorous acid 7.46 HBrO Hypobromous acid 8.72 NH4+ Ammonium 9.26 HCN Hydrogen cyanide 9.36 HIO Hypoiodous acid 10.66
STOICHIOMETRY ACID-BASE EQUILIBRIA
A) Boiling Point, Melting Point and Enthalpy of Vaporisation Enthalpy of vaporisation – the heat energy required to convert 1 mol of a liquid to its vapour at the boiling point of the liquid. Example: Period 2 (Li, Be, B, C, N, O, F, Ne) & Period 3 (Na, Mg, Al, Si, P, S, Cl, Ar) Across the period: the element become less metallic. Li, Be, Na, Mg and Al are metals (metal lattice): B, C (graphite) and Si are metalloids & C (diamond) is non-metal (giant covalent molecule): N, O, F, P, S, Cl are non-metallic elements (simple molecular structure): Ne and Ar are non-metallic (monoatomic structure): B) Electrical Conductivity
Atomic orbital – the region, or volume, of space in an atom within the high probability (95% chance) of finding an electron in an atom. Types of orbital: s, p, d and f orbital Core shell – first shell that holds two electrons Valence shell – the outermost shell Effective nuclear charge (Nuclear attraction) accounts for (increases from left to right of the periodic table): Ionisation energy is influenced by: Electronic Structure Number of electrons in shell = 2(n)2 Nucleus = made up of both neutrons and protons Arrangement of electrons in an atom Electronic Configurations The electrons are filled according the orbitals (Aufbau principle). (Extended knowledge: the process is repeated until all of the electrons have been accounted for. g-, h- and j-orbital exist in theory but the periodic table contains no elements that have electrons in either g-, h- and j-orbitals.) The first break from numerical sequencing comes when the 4s level is filled before the 3d level, despite the fact that the perimeter of the 3d level is closer to the nucleus than the 4s orbital. The reason is that the energy of the level is based on an average position of the electron, not the extreme position. Ionising electrons are not removed from the atom in reverse order! However, the outer shell electrons are always removed first when forming cations. Examples Example 1: Electronic configuration for manganese. -> Solution 1: Example 2: Which column of the periodic table is diamagnetic? -> Solution 2: Example 3: Electronic configuration for chromium -> Solution 3: Example 4: Electronic configuration for copper. -> Solution 4: Example 5: Which of the following electronic configuration represents an exited state? -> Solution 5: Important: Not to confuse an ion (either cation or anion) with an excited state. A cation is an atom that has a deficit of at least one electron and thus carries a positive charge. An anion is an atom that has an excess of at least one electron and thus carries a negative charge. Periodic Table can be classified into 4 main groups. 1) The s-block elements: 2) The p-block elements 3) The d-block elements 4) The f-block elements
Core shell = 1st energy level (electron occupancy of 2)
Valence shell = 2nd energy level (electron occupancy up to 8 )
A. He: 1s2
B. Li: 1s22p1
C. N: 1s22s22p3
D. F: 1s22s22p6
Periodicity of Atomic Radius Atomic radii for elements in Periods 2 and 3 Atomic radii can be classified into three categories: Effecting factors of the atomic radius: A) Atomic radius across a period Example: Period 2 (Li, Be, B, C, N, O, F, Ne) and Period 3 (Na, Mg, Al, Si, P, S, Cl, Ar) Across the period: B) Atomic radius down a group Example: Group 2 (Be, Mg, Ca, Sr, Ba) Down the group: C) Ionic radius (radius of a cation or or an anion) across Period 3 Isoelectronic – species have the same number of electrons and the same electronic configuration. When given number of electrons (Na+, Mg2+, Al3+) or (P3-, S2-, Cl-) When given nuclear charge, Conclusion: D) Ionic radius down a group Example: Group 2 (Be2+, Mg2+, Ca2+, Sr2+, Ba2+) & Group 17 (F-, Cl-, Br-, I-) Going down the Group 2 and Group 17:Elements Atomic radius (pm) Li 152 Be 112 B 80 C 77 N 74 O 74 F 72 Na 156 Mg 136 Al 125 P 110 S 104 Cl 99 Ion Ionic radius No. of electrons No. of protons Na+ 0.095 10 11 Mg2+ 0.065 10 12 Al3+ 0.050 10 13 P3- 0.212 18 15 S2- 0.184 18 16 Cl- 0.181 18 17
A) Electronegativity
Electronegativity – measure how easy it is for an atom to gain electrons and how much an atom will pull electrons away from other atoms it has bonded to / covalent bond (similar to electron affinity but the difference is electron affinity deals with isolated atoms in the gas phase).
Across the periodic table (left to right)
Electronegativity increases.
Left side: prefer to lose electrons.
Right side: prefer to gain electrons.
Noble gases: no electron affinity.
Down the periodic table
Electronegativity decreases.
It is because the shielding effect (nuclear charge increases but screening effect increase and the atomic size increases and as a result, the effective charge decrease).
B) Electron Affinity
Electron affinity – the energy change that occurs when a gaseous atom picks up an extra electron.
First electron affinity is exothermic:
Example: O (g) + e –> O- (g)
First electron is pulled/attracted by the positively charged oxygen atom nucleus.
Second electron affinity is endothermic:
Example: O- (g) + e –> O2- (g)
Second electron is repelled by the existing negative charge on the oxygen ion.
Across the periodic table (left to right)
Left: elements want to lose electrons to be the nearest noble gas. As result, not much energy is released when these elements gain an extra electron. Electron affinity to be slightly negative.
Right: elements want to gain electrons to be the nearest noble gas. As result, a very high energy to be released. Electron affinity to be more negative.
Down the periodic table
Elements want to gain electron less (shielding effect)
Bottom: elements have less negative electron affinities.
C) Variation of the Period of d-block Element
Across the periodic table – First series (left to right)
Atomic size is approximately the same (except Sc and Ti).
Effective nuclear charge remains almost.
High melting points and boiling points (except Zn).
Density increases (but decreases for zinc).
1st and 2nd ionisation energies of the elements increase slightly (as the proton numbers increase)
3rd and 4th ionisation energies of the elements increase drastically.
Checking whether i can rmb anot Cell Mediated :(
Cell Mediated.
Part 1
1. Viral infection
2. Macrophage engulf,ingest the pathogen (not completely).
3. Some fragment of the foreign antigen bought up to macrophage surface and combine to the MHC 2-protein which is located at the cell surface.
4. Macrophage become the APC (Antigen presenting cell) Whole thing call antigen MHC 2 complex
Part 2
1. Helper T cell will cognated with the Complex with aids of coreceptor CD4+
2. The complex and Helper T will be hold together by CD4.
3. This activates the complex (APC)and stimulates the secretion of IL 1 (cytokines)
4. IL 1 will activates the Helpher T to divide and increase in size,and Helper T will produce IL 2.
5. IL 2 in turns stimulates more production of Helper T cells and even more interleukins produced by Helper T.
IL 2 also stimulates the NK cells and B-cells.
IL 2 also stimulates the poliferation of Cytotoxic T cells
IL 2 also stimulates Cytotoxic T to release perforin
Part 3
1. Cytotoxic T cells have coreceptor CD8+ will recognize the viral antigen on MHC 1-protein.
2. Perforin is released. by Cytotoxic T.
3. Perforin cre8 holes,pores on target cell's membrane. causes lysis to happen,burst or shrink whatever and destroyed
4. Antibodies circulating the virus will destroy the viral cell
Humoral response
Part 1
1. Viral infection
2. Macrophage engulf,ingest the pathogen (not completely).
3. Some fragment of the foreign antigen bought up to macrophage surface and combine to the MHC 2-protein which is located at the cell surface.
4. Macrophage become the APC (Antigen presenting cell) Whole thing call antigen MHC 2 complex
Part 2
1. Helper T cell will cognated with the Complex with aids of coreceptor CD4+
2. The complex and Helper T will be hold together by CD4.
3. Helper T start to divide and activated
Part 3
1. B-cells with MHC 2 on their surface will displace T-dependent antigen.
2. B-cell become APC
3. Activated helper T on step 3 part 2 will recognize the antigen being displaced bcz same antigen source.
4. the binding will stimualates the release of IL 2.
5. IL 2 will stimulate B-cell to poliferate.
6. Plasma B-cells produce numerous clones antibodies. and to the bloodstream and body fluid and to the infection site.
7. Memory B-cells for future use,if and only if exposed to same pathogen attack
End.
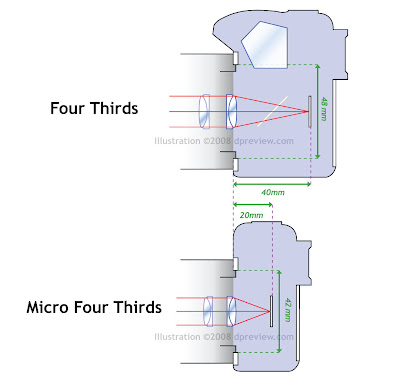
Olympus and Panasonic announced the new, mirrorless format / lens mount based on (and compatible with) Four Thirds in August of last year. The Micro Four Thirds system uses the same sensor size (18 x 13.5 mm) but allows slimmer cameras by removing the mirror box and optical viewfinder. The new format has three key technical differences: (1) roughly half the flange back distance (distance from mount to the sensor), (2) a smaller diameter lens mount (6 mm smaller) and (3) two additional contact points for lens-to-body communication (now 11 points). Removing the mirror mechanism allows this shorter flange back distance, meaning lenses for the new mount can be considerably smaller than current Four Thirds designs. The format requires framing to be carried out using Live View on either the LCD monitor or an EVF. Existing Four Thirds lenses can be used on Micro Four Thirds cameras using an adapter.


EP 1 超Retro lorr . Racun jor
From the OM system 35mm SLRs and lenses to the XA series rangefinders and the half-frame Pen models, Olympus has for at least half a century been notable for producing cameras that are smaller than their competitors without sacrificing quality or functionality. And they haven't stopped; the E-450 and its predecessors are still the world's smallest digital SLRs, and the new E-620 is considerably smaller than similarly specified competitors (finally realizing the 'smaller format, smaller camera' promise we were all sold on when Four Thirds originally launched).
The apex of this miniaturization was surely the Pen F and its variants - the interchangeable lens versions of the hugely popular Pen series (over 17 million of the various models were sold between 1959 and the mid 80's when half-frame finally died out). A fully-fledged single lens reflex camera that was smaller than most rangefinders (thanks to its half frame film format), the Pen F was innovative, it was stylish and, in 1963 when it was launched, it was universally lauded, and 45 years later enjoys true classic status.
The first Micro Four Thirds camera from Olympus pays unabashed homage to the Pen F; from the classic styling to the long running teaser campaign running in print and online, the E-P1 doesn't just wear its influences on its sleeve; it shouts about them from the rooftops (and is referred to in some parts of the world as the 'Digital Pen'). There's even a subtle engraving on the chrome edge of the top plate that reads 'Olympus Pen Since 1959'.


But 过下 Leica 瘾 , + the cheapest among three.
recently especially approaching stpm ,saw lotsa freakin' dude declare emself AWESOME talk,blog,advertise *yeah ADVERTISE* their wishes,dreams,abilities *even they don't have it* like they're gonna be the pioneer of the society soon bla bla bla.. HAHA sound so funny. End.


I'm a MANU hater. Fuck em' ass hole ferguuuson.. But #5 by Rio Ferdinand ,nontheless is superb. Take a look :) it's FOC

There is no mystery about who this person is. There is also no mystery about how the Weimar Republic came to an end. As people saw, things continued to get worse and worse and worse in the Weimar Republic. The currency was worthless, there were no jobs, and the people were cold and hungry. Then a brilliant orator entered onto the stage, and he promised to bring Germany back to its former glory. He had a nice "list" of people and groups responsible for Germany's problems. He appealed to a sense of German Nationalism and pride. The story ends in another World War, and countless millions of innocent people being exterminated. for him? i've got no comment. night peeps xoxo
well i'm not a racist. i fuck racism too. but please no matter laa mat7 race you are. as long as ure a human. act like a human,be polite and do ur job properly. terutamanya ure a gov. officer NIAMA Kakitangan kerajaan means? Means ure representing our country laa Sigh if u org pny keagamaan can't fix u. Come Pendidikan Moral and Sivik is always here for you. I TEACH YOU.
I don't know. since when we can't even talk to each other anymore.. everytime i see you my heart pain a lil while. I told you ,I din't did it on purpose. it's an accident. The most syok part is, i don't even have the courage to talk to you ,Its not because of guilty of whatso'eva


i blog it out because it is super easy and short.
haha. too boring larr actually. well lets proceed ,
- its a natural cycling process,dynamic system means can be disturbed by human activities and i would summarise it as pollutions.
-fluxes of macronutrients lorr. the reason geologist study on these element is because of their biological well-being. i presume u guys know their importance to us respectively. LOL
-things to take note about the flowing of these essential element
Firstly,various form of these nutrients (inorganic ions or element la of cz) flows from the non-living(abiotic) large reservoir pool e.g. sedimentary for phosphorus on ocean floors/fluid for carbon and nitrogen to living component(biotic) ,the small active/cycling pool by a few ways which will be seen later. And back to the abiotic pool again. :)
Dimana the transfer of these chemicals in biological pathway and through geological processes will form a cycle keep on flow and flow and flow~ It happens in biosphere(sum and interaction between all ecosystem)
1.Interactions btw biotic and abiotic
2.Role of Decomposers
3.The Processes and importance of these nutrients to us
4.Strategy haha cheh kononnya
If essay come out. I'll draw the flow chart out 1st. thn elaborate frm the chart (most secure way) :)
Locate the source(reservoir pool) and the living component(active pool) then memorise those stupid but essential bacteria's scientific name P/S its a MUST *sigh*
Carbon cycle and Hydro Cycle is not important so i'll omit em' (if this year come out these 2 cycle i potong!) LOL
I know in words form vry hard to understand but nvm laarr since i so sien wtf i'll cut short and write main point
Phosphorus Cycle first larr. erm phosphorus is the essential component for nucleic acid (RNA DNA) , ATP,phospholipids,proteins etc..
Inorganic phosphate in rocks will erode and leach to freshwater,oceans(some dissolve in soil). dekat situ,those aqua organism will actively take in these inorganic phosphate. Lepas tu,they will die and decomposition takes place. then when they die,their corpse will sink to the ocean floor and sedimentation happen. henceforth a reservoir pool of inorganic phosphate is build on ocean floor ;) hundreds thousands yrs ltr. geological lands uplift will bring those inorganic phosphate up and they are now found in the rocks!
For those inorganic phosphate in rocks tat erode to soil,or by mining activities/industrial production of fertilisers ,it(inorg phosphate) will dissolve. As a result,active ions uptake by plant roots will occur and by assimilation,Organic phosphate is produced in plants and plants will die and then decay by decomposers or eaten by herbivores up food chain and they die and decompose by decomposer(lack of the decomposer info,anyone know plz tell me tq).
Excretory waste materials and shells bones teeth of animals oso will be decompose.
Sulphur Cycle,
this one more fun,3 sources(SO2 in atmosphere,Hydrogen sulphide,H2S in soil,elementary Sulphur in rocks*the reservoir pool*) and 1 center(Sulphate ion,SO4 2-)
H2S derived from the decomposition of death animal corpse,faeces and plants by the anaerobic reducing bacteria,Desulphovibrio. H2S will be reduced to Sulphur by photosynthetic bacteria Chlorobium and Chromatium.
The Sulphur can either incorporated into rock to build up the reservoir pool OR will be oxidize by chemoautotrophic sulphur bacteria,Thiobacillus to form Sulphate ion,SO4 2-
The Sulphur in rocks will become SO2 in atmosphere by volcano eruption,mining and combustion of fuels. Oh ya i forgot 1,by industrial processes(fixation) to produce sulphate fertiliser. (inside those fertiliser gt sulphate ions) tats y fertiliser is needed ==.
The Sulphur dioxide will thn change to Sulphate ions by percipitation of acid rain.
Lastly,phew(so freaking sleepy alr) the sulphate ions will be absorbed by roots of plant then incorporate in plant protein(in R-SH form) then plant die decompose by Desulphovibrio OR plant eaten by animal (also in R-SH form it stores)thn animal mampus and excrete waste product thn decomposer start working and all,change back to HYDROGEN SULPHIDE.
oh ya,sulphur is important to us once it transform to SH group and this SH group is used to synthesise coenzyme A,Biotin and one of the essential amino acid,Cysteine
Easy,just rmb Nitrogen gas,ammonium,nitrite,nitrate and nitrogen fixation will do.
Take Note,3 types of fixations. 2 naturally 1 by Haber process(refer inorganic chem or form 5 larr)
OR ,by industrial(Haber) process to produce fertiliser and dlm tu got ammonium and nitrate.
OR, by Rhizobium,super famous nitrogen fixing bacteria .__. thn fix to ammonia and glutamine and is found in protein in plant. then same again,plant die,decomposed or feed by animal animal excrete urea and die decay all become Ammonium,NH4+
Nitrification by Nitrosomonas and Nitrobacter bla bla..frm NH4+ -- NO2 (nitrite) --- NO3(nitrate) Of cz its a oxidation process larr. then denitrification by denitrifying bacteria,Thiobacillus denitrificans and convert to nitrogen gas again.The Nitrate oso will be actively uptake by plant root.
Lastly ,happening in soil. Free living bacteria,Nostoc and Azotobacter die and decay and undergo ammonification which is,i get from wiki.. all books oso xrak .. Bacteria, or in some cases, fungi, convert the organic nitrogen within the remains back into ammonium (NH4+) . So completed the nitrogen cycle sudah. Semoga ni kluar stpm thn ni ahaaa.
xoxo
I kinda hate mah phone. Pc suite is totally usefulnessless for me. why? because i can't transfer my inbox msgs to mah laptops ITS FULL i can't receive msgs yet I can't delete those msgs ,I can't erase em' like they doesn't exist. I can't stop mahself for living in past. Too many I can't. I can't get over you. Imma be those particles in black hole. No More Light








